Anticoagulant Sodium Citrate 4%
Anticoagulant Sodium Citrate 4% is intended for extracorporeal use as anticoagulant for whole blood in automated apheresis procedures. Not for direct intravenous infusion.
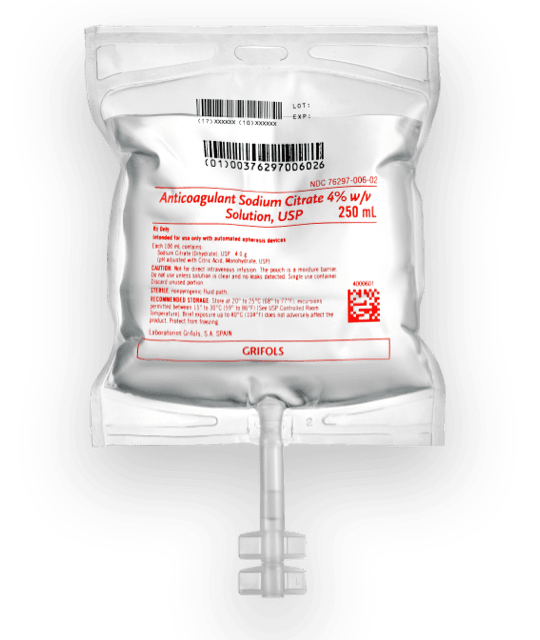

Product overview
- Anticoagulant Sodium Citrate 4% is supplied in flexible polypropylene (PP) plastic bags containing 250 mL of solution.
- The container is free of PVC, plasticizers, adhesives, and latex.
- The solution is aqueous-based, clear, and colorless.
Designed for safe and easy handling
Integrated eyelet support for easy and safe handling of the container during use.
Product information
- Inclusion of the National Drug Code
- Inclusion of lot and expiration date
- Sequential number
Highly compatible material
High resistance pressure cuffs respond satisfactorily to 400 mmHg pressure for 72 hours.
Outlet port
PP tube closed with a PP twist-off for connecting to the apheresis device.
Guidelines for Anticoagulant Sodium Citrate 4%
Overwrap removal
The overwrap serves as a moisture barrier. It is intended to limit evaporative moisture loss from the primary solution container.
- The overwrap is designed to be opened by pulling apart the 2 sheets of the overwrap.
- The recommended method to remove the overwrap is to peel off one sheet by the corner while holding the other sheet at one end to carefully remove the solution container.
The overwrap should not be removed until the product is to be used.
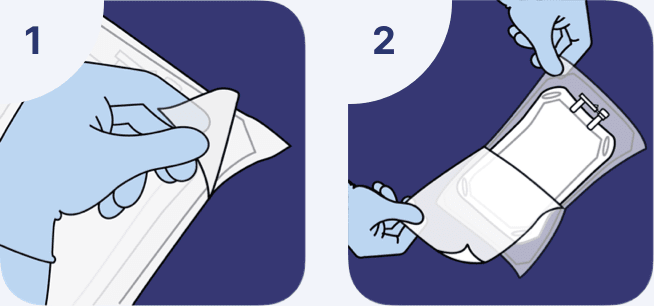
Container inspection
- Visually inspect the container for particulate matter and discoloration
- Check for minute leaks by squeezing the inner container firmly. If leaks are found, discard the solution as sterility may be impaired
- If the ports are damaged, detached, or not present, discard the container as solution path sterility may be impaired
Do not administer unless the solution is clear and seal is intact.
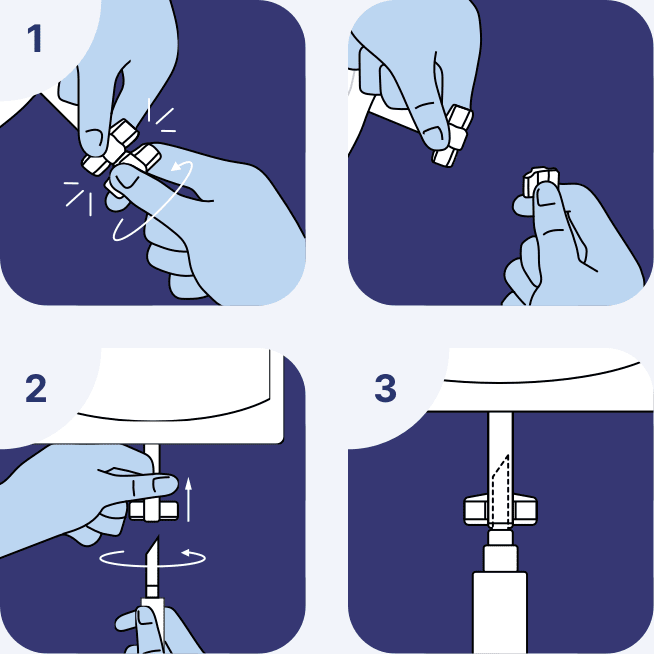
Attach administration set
- Open the bag's breakable access port.
- Hold the container properly and aseptically connect the bag to the anticoagulant line of the apheresis device set. Completely insert the spike of the apheresis device tubing set through the port as far as it will go. To ensure proper connection and prevent leakage, push the set using a circular motion up to the shoulder of the spike. Do not move the spike back and forth once inserted.
- Verify that the bag has been securely attached to the anticoagulant line of the system's tubing set to avoid flow interruption, leaks, and
connection errors.
Delivery information
Grifols ships our Anticoagulant Sodium Citrate 4% with a minimum order qualtity of a full sea container:
- Sea container (40 ft high cube reefer) contains: 46 EU pallets double-stacked
- EU pallet contains: 32 carton box (8 boxes per layer/4 layers)
Anticoagulant Sodium Citrate
4% 250 mL
- NDC Number
-
76297-006-02
- Case weight Kg
-
8.5
- Bags/ box
-
39
- Bags/ pallet
-
1,248
- Bags/ container
-
57,408
| Description | NDC Number | Case weight Kg | Bags/ box | Bags/ pallet | Bags/ container |
|---|---|---|---|---|---|
|
Anticoagulant Sodium Citrate |
76297-006-02 |
8.5 |
39 |
1,248 |
57,408 |
Important Safety Information
LABORATORIOS GRIFOLS SA
Rx only
250 mL
Intended for use only with automated apheresis devices.
Each 100 mL contains:
Sodium Citrate (Dihydrate), USP 4 g (pH adjusted with Citric Acid, Monohydrate, USP)
Not for direct intravenous infusion. The pouch is a moisture barrier. Do not use unless solution is clear and no leaks detected. Single use container. Discard unused portion.
Store at 20 °C to 25 °C (68 °F to 77 °F); excursions permitted between 15 °C to 30 °C (59 °F to 86 °F)
[See USP Controlled Room Temperature]. Brief exposure up to 40 °C (104 °F) does not adversely affect the product. Protect from freezing.
SPAIN
Review: 10/2019
Do you have any questions or concerns?
Contact a Grifols representative for more information