0.9% Sodium Chloride Injection, USP, in Fleboflex plastic container
Partners in excellence
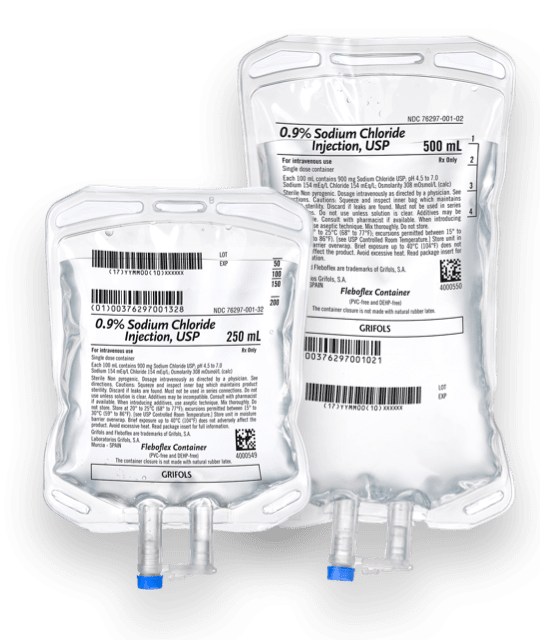
Delivering a safe, reliable, and convenient IV solution
0.9% Sodium Chloride Injection, USP, is a sterile, nonpyrogenic solution for fluid and electrolyte replenishment in the Fleboflex® plastic container for intravenous administration.
Product overview
- Fleboflex® is a flexible container free of PVC, plasticizers, adhesives, and latex
- The Fleboflex® container consists of a polypropylene multilayer film
- Polypropylene is a highly compatible material. It is used for the preparation of intravenous mixtures with drugs that have shown their incompatibility with other plastics
- Fleboflex® is totally collapsible, lightweight, and transparent
- The Fleboflex® container meets the Class VI US Pharmacopeia (USP) testing for plastic containers. These tests confirm the biological safety of the container system
- Available in 50, 100, 250, 500, and 1000 mL
Max. additive volume in mL up to a pressure of 50 mL bar
- 50 mL
-
90
- 100 mL
-
88
- 250 mL
-
135
- 500 mL
-
288
- 1000 mL
-
176
Maximum removable volume (mL)
- 50 mL
-
145
- 100 mL
-
195
- 250 mL
-
397
- 500 mL
-
716
- 1000 mL
-
1207
Residual volume (mL)
- 50 mL
-
0-1
- 100 mL
-
0.3
- 250 mL
-
0.6 - 1.6
- 500 mL
-
0.6 - 7.1
- 1000 mL
-
0.6 - 5
Air volume (mL)
- 50 mL
-
4-10
- 100 mL
-
5-12
- 250 mL
-
5-15
- 500 mL
-
12-25
- 1000 mL
-
15-25
| Fleboflex® | 50 mL | 100 mL | 250 mL | 500 mL | 1000 mL |
|---|---|---|---|---|---|
|
Max. additive volume in mL up to a pressure of 50 mL bar |
90 |
88 |
135 |
288 |
176 |
|
Maximum removable volume (mL) |
145 |
195 |
397 |
716 |
1207 |
|
Residual volume (mL) |
0-1 |
0.3 |
0.6 - 1.6 |
0.6 - 7.1 |
0.6 - 5 |
|
Air volume (mL) |
4-10 |
5-12 |
5-15 |
12-25 |
15-25 |
Compatibility Studies1
Fleboflex® has drug-container compatibility with a select number of drugs*
1. Internal studies elaborated by Grifols (v1.0) - all document shares internal studies elaborated by grifols (p.1)
Cefotaxime
- Composition
-
0.5 mg/mL in 0.9% SC
- 5 °C (41 °F)†
-
10 days
- 25 °C (77 °F)†
-
1 days
Cefuroxime
- Composition
-
0.5 mg/mL in 0.9% SC
- 5 °C (41 °F)†
-
10 days
- 25 °C (77 °F)†
-
1 days
Cyclophosphamide
- Composition
-
2 mL in 0.9% SC
- 5 °C (41 °F)†
-
6 days
- 25 °C (77 °F)†
-
5 days
Diazepam
- Composition
-
50 μg/mL in 0.9% SC
- 5 °C (41 °F)†
-
10 days
- 25 °C (77 °F)†
-
4 days
Docetaxel
- Composition
-
0.3 mg/mL in 0.9% SC
- 5 °C (41 °F)†
-
7 days
- 25 °C (77 °F)†
-
7 days
Doxorubicin
- Composition
-
0.1 mg/mL in 0.9% SC
- 5 °C (41 °F)†
-
30 days
- 25 °C (77 °F)†
-
15 days
Etoposide
- Composition
-
0.3 mg/mL in 0.9% SC
- 5 °C (41 °F)†
-
-
- 25 °C (77 °F)†
-
10 days
5-fluorouracil
- Composition
-
3 mg/mL in 0.9% SC
- 5 °C (41 °F)†
-
28 days
- 25 °C (77 °F)†
-
28 days
Isosorbide Dinitrate
- Composition
-
0.1 mg/mL in 0.9% SC
- 5 °C (41 °F)†
-
10 days
- 25 °C (77 °F)†
-
10 days
Methylprednisolone
- Composition
-
5 mg/mL in 0.9% SC
- 5 °C (41 °F)†
-
10 days
- 25 °C (77 °F)†
-
Free Prednisolone >6.6 days
Morphine
- Composition
-
0.1 mg/mL in 0.9% SC
- 5 °C (41 °F)†
-
10 days
- 25 °C (77 °F)†
-
10 days
Nitroglycerin
- Composition
-
50 μg/mL in 0.9% SC
- 5 °C (41 °F)†
-
10 days
- 25 °C (77 °F)†
-
10 days
Ondansetron
- Composition
-
1 mg/mL in 0.9% SC
- 5 °C (41 °F)†
-
10 days
- 25 °C (77 °F)†
-
10 days
Paclitaxel
- Composition
-
0.6 mg/mL in 0.9% SC
- 5 °C (41 °F)†
-
7 days
- 25 °C (77 °F)†
-
7 days
| Drug | Composition | 5 °C (41 °F)† | 25 °C (77 °F)† |
|---|---|---|---|
|
Cefotaxime |
0.5 mg/mL in 0.9% SC |
10 days |
1 days |
|
Cefuroxime |
0.5 mg/mL in 0.9% SC |
10 days |
1 days |
|
Cyclophosphamide |
2 mL in 0.9% SC |
6 days |
5 days |
|
Diazepam |
50 μg/mL in 0.9% SC |
10 days |
4 days |
|
Docetaxel |
0.3 mg/mL in 0.9% SC |
7 days |
7 days |
|
Doxorubicin |
0.1 mg/mL in 0.9% SC |
30 days |
15 days |
|
Etoposide |
0.3 mg/mL in 0.9% SC |
- |
10 days |
|
5-fluorouracil |
3 mg/mL in 0.9% SC |
28 days |
28 days |
|
Isosorbide Dinitrate |
0.1 mg/mL in 0.9% SC |
10 days |
10 days |
|
Methylprednisolone |
5 mg/mL in 0.9% SC |
10 days |
Free Prednisolone >6.6 days |
|
Morphine |
0.1 mg/mL in 0.9% SC |
10 days |
10 days |
|
Nitroglycerin |
50 μg/mL in 0.9% SC |
10 days |
10 days |
|
Ondansetron |
1 mg/mL in 0.9% SC |
10 days |
10 days |
|
Paclitaxel |
0.6 mg/mL in 0.9% SC |
7 days |
7 days |
Bag features

Designed for safe and easy handling
- Designed with rounded upper and lower corners that guarantee handling without accidental punctures
- Integrated eyelet support for easy and safe handling of the container during the infusion
Highly compatible material
- The solution is only in contact with polypropylene. Both the multilayer film and the inner membranes of the medication and outlet ports contain only polypropylene
- Polypropylene can be sterilized at a higher temperature because it resists heat better than other olefins
High sealing resistance
High resistance to pressure cuffs, responding satisfactorily to 400 mmHg pressure for 72 hours.
Product information
- Inclusion of the National Drug Code
- Inclusion of lot and expiration date
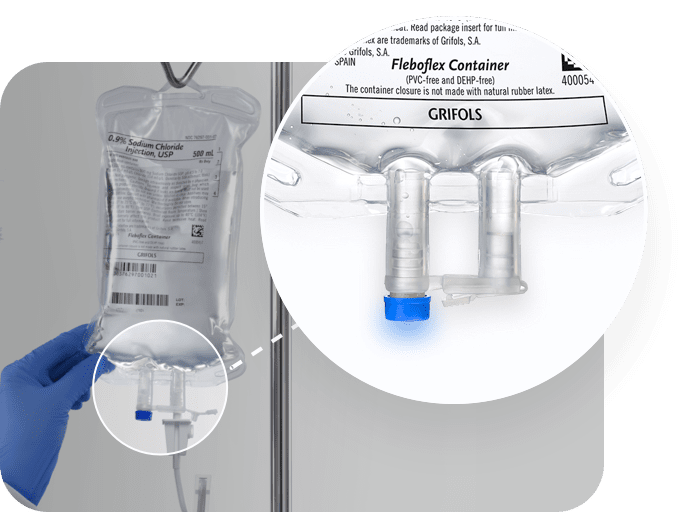
Grifols port system
- Medication and outlet ports designed with rigid and long tubes to avoid perforation due to needle insertion
- Safe attachment of the infusion set due to its internal membrane
- No parts of the cover have to be removed in order to access the outlet port
Individual overwrap of polypropylene protects and maintains the sterility of the container and limits evaporative moisture loss from the primary solution container. It is transparent to allow visual inspection and has a peelable opening system.
Product also available in Canada
Delivery information
Grifols ships our 0.9% Sodium Chloride Fleboflex® Luer bags with a minimum order qualtity of a full sea container:
- Sea container (40 ft high cube reefer) contains: 46 EU pallets double-stacked
- EU pallet contains: 32 carton box (8 boxes per layer/4 layers)
0.9% Sodium Chloride Injection,
USP 50 mL
- NDC Number
-
76297-001-11
- Case weight Kg
-
8
- Bags/ box
-
115
- Bags/ pallet
-
3680
- Bags/ container*
-
169,280
0.9% Sodium Chloride Injection,
USP 100 mL
- NDC Number
-
76297-001-21
- Case weight Kg
-
8.5
- Bags/ box
-
70
- Bags/ pallet
-
2240
- Bags/ container*
-
103,040
0.9% Sodium Chloride Injection,
USP 250 mL
- NDC Number
-
76297-001-31
- Case weight Kg
-
8.5
- Bags/ box
-
28
- Bags/ pallet
-
896
- Bags/ container*
-
41,216
0.9% Sodium Chloride Injection,
USP 500 mL
- NDC Number
-
76297-001-01
- Case weight Kg
-
11.6
- Bags/ box
-
20
- Bags/ pallet
-
640
- Bags/ container*
-
29,440
0.9% Sodium Chloride injection,
USP 1000 mL
- NDC Number
-
76297-001-41
- Case weight Kg
-
11
- Bags/ box
-
10
- Bags/ pallet
-
320
- Bags/ container*
-
14,720
| Description | NDC Number | Case weight Kg | Bags/ box | Bags/ pallet | Bags/ container* |
|---|---|---|---|---|---|
|
0.9% Sodium Chloride Injection, |
76297-001-11 |
8 |
115 |
3680 |
169,280 |
|
0.9% Sodium Chloride Injection, |
76297-001-21 |
8.5 |
70 |
2240 |
103,040 |
|
0.9% Sodium Chloride Injection, |
76297-001-31 |
8.5 |
28 |
896 |
41,216 |
|
0.9% Sodium Chloride Injection, |
76297-001-01 |
11.6 |
20 |
640 |
29,440 |
|
0.9% Sodium Chloride injection, |
76297-001-41 |
11 |
10 |
320 |
14,720 |
Guidelines for use of 0.9% Sodium Chloride solution in Fleboflex® container
Overwrap removal
- The overwrap is designed to be opened by pulling apart the 2 sheets of the overwrap.
- The recommended method to remove the overwrap is to peel off one sheet by the corner while holding the other sheet at one end to carefully remove the solution container.
The overwrap should not be removed until product is to be used.
To add medication
- Prepare the medication site. Using a syringe with a 19- to 22-gauge needle, securely hold the container and insert the needle through the center of the medication port and inject the medication. Insert the needle perpendicular to the point of addition.
The compatibility of the additives with Sodium Chloride 0.9% must be checked before adding a medication.
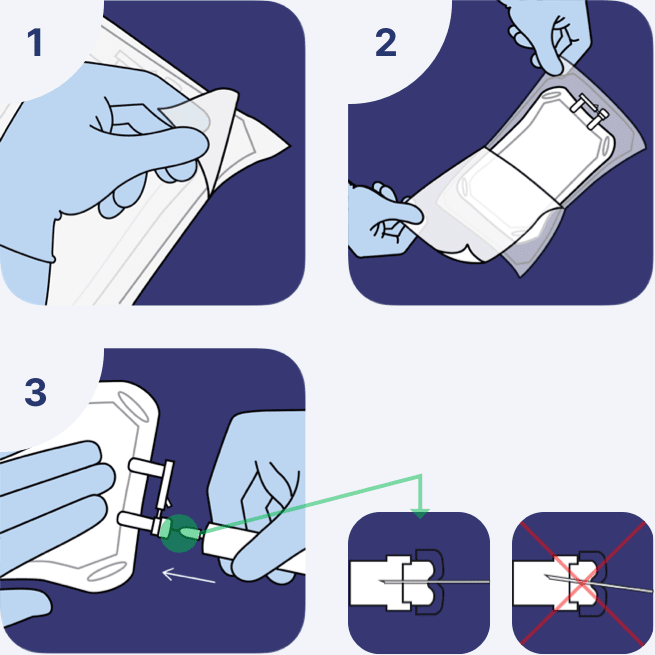
Attach administration set
- Suspend the container from the eyelet support and remove the protector from the outlet port at the bottom of the container.
- Hold the container properly and attach the administration set.
- While advancing through the tube, a slight resistance will gradually be felt.
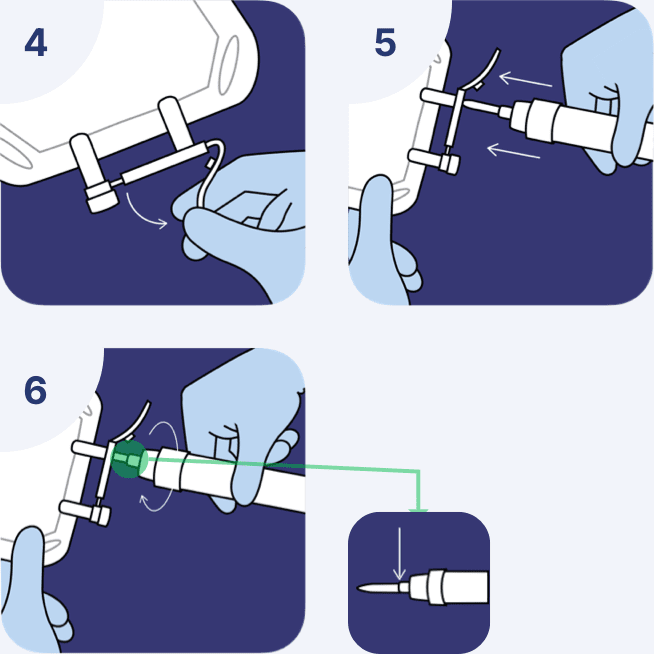
Use non-vented administration sets
The Fleboflex® container has been designed to work without venting. No air is required to withdraw the solution.
Air entering through the administration set could slow or even stop the infusion due to air bubbles.
- After opening the container, the contents should be used immediately and should not be stored for a subsequent infusion. Do not reconnect any partially used containers. Discard any unused portion.
Administration sets should come without air venting. If vented administration sets are used, be sure air venting inlet remains closed during the infusion.
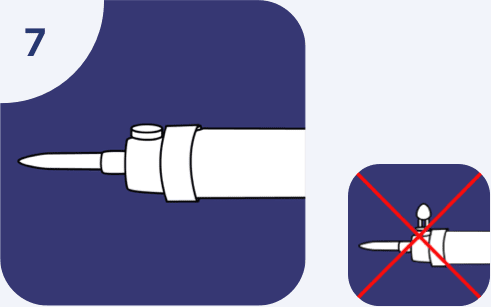
Do not connect flexible plastic containers in a series in order to avoid air embolism due to possible residual air contained in the primary container. Using a vented intravenous administration set with the vent in the open position could result in air embolism. Vented intravenous administration sets with the vent in the open position should not be used with flexible plastic containers.
Important Safety Information
Sodium Chloride Injection, USP is indicated as a source of water and electrolytes. 0.9% Sodium Chloride Injection, USP is also indicated for use as a priming solution in hemodialysis procedures.
Warnings
Hypersensitivity
Hypersensitivity and infusion reactions, including hypotension, pyrexia, tremor, chills, urticaria, rash, and pruritus have been reported with 0.9% Sodium Chloride Injection, USP.
Stop the infusion immediately if signs or symptoms of a hypersensitivity reaction develop, such as tachycardia, chest pain, dyspnea and flushing. Appropriate therapeutic countermeasures must be instituted as clinically indicated.
Electrolyte Imbalances
Fluid Overload
Depending on the volume and rate of infusion, and the patient’s underlying clinical condition, the intravenous administration of Sodium Chloride Injection, USP can cause fluid disturbances such as overhydration/hypervolemia and congested states, including pulmonary congestion and edema.
Avoid 0.9% Sodium Chloride Injection, USP in patients with or at risk for fluid and/or solute overloading. If use cannot be avoided, monitor fluid balance, electrolyte concentrations, and acid base balance, as needed and especially during prolonged use.
Hyponatremia
Sodium Chloride Injection, USP may cause hyponatremia. Hyponatremia can lead to acute hyponatremic encephalopathy characterized by headache, nausea, seizures, lethargy, and vomiting. Patients with brain edema are at particular risk of severe, irreversible and lifethreatening brain injury. Avoid Sodium Chloride Injection, USP in patients with or at risk for hyponatremia. If use cannot be avoided, monitor serum sodium concentrations.
Hypernatremia
Hypernatremia may occur with Sodium Chloride Injection, USP. Conditions that may increase the risk of hypernatremia, fluid overload and edema (central and peripheral), include patients with: primary hyperaldosteronism; secondary hyperaldosteronism associated with, for example, hypertension, congestive heart failure, liver disease (including cirrhosis), renal disease (including renal artery stenosis, nephrosclerosis); and pre-eclampsia. Avoid Sodium Chloride Injection, USP in patients with, or at risk for, hypernatremia. If use cannot be avoided, monitor serum sodium concentrations.
Precautions
Patients with Severe Renal Impairment
Administration of Sodium Chloride Injection, USP in patients with or at risk of severe renal impairment, may result in hypernatremia and/or fluid overload. Avoid Sodium Chloride Injection, USP in patients with severe renal impairment or conditions that may cause sodium and/or potassium retention, fluid overload, or edema. If use cannot be avoided, monitor patients with severe renal impairment for development of these adverse reactions.
Drug Interactions
Other Products that Affect Fluid and/or Electrolyte Balance
Administration of Sodium Chloride Injection, USP to patients treated concomitantly with drugs associated with sodium and fluid retention may increase the risk of hypernatremia and volume overload. Avoid use of Sodium Chloride Injection, USP in patients receiving such products, such as corticosteroids or corticotropin. If use cannot be avoided, monitor serum electrolytes, fluid balance and acid-base balance.
Lithium
Renal sodium and lithium clearance may be increased during administration of 0.9% Sodium Chloride Injection, USP. Monitor serum lithium concentrations during concomitant use.
Other Drugs that Increase the Risk of Hyponatremia
Administration of Sodium Chloride Injection, USP in patients treated concomitantly with medications associated with hyponatremia may increase the risk of developing hyponatremia.
Avoid use of Sodium Chloride Injection, USP in patients receiving products, such as diuretics, and certain antiepileptic and psychotropic medications. Drugs that increase the vasopressin effect reduce renal electrolyte free water excretion and may also increase the risk of hyponatremia following treatment with intravenous fluids. If use cannot be avoided, monitor serum sodium concentrations.
Pregnancy
Sodium Chloride Injection, USP should be given during pregnancy only if the potential benefit justifies the potential
risk to the fetus.
Lactation
It is not known whether this drug is present in human milk. Because many drugs are present in human milk, caution should be exercised when Sodium Chloride Injection, USP is administered to a nursing woman.
Pediatric Use
Closely monitor plasma electrolyte concentrations in pediatric patients who may have impaired ability to regulate fluids and electrolytes. In very low birth weight infants, excessive or rapid administration of Sodium Chloride Injection, USP may result in increased serum osmolality and risk of intracerebral hemorrhage.
Children (including neonates and older children) are at increased risk of developing hyponatremia as well as for developing hyponatremic encephalopathy.
Geriatric Use
Geriatric patients are at increased risk of developing electrolyte imbalances. Sodium Chloride Injection, USP is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function.
Adverse Reactions
The following adverse reactions have been reported in the post-marketing experience during use of 0.9% Sodium Chloride Injection, USP and include the following: General disorders and administration site conditions (Infusion site erythema, injection site streaking, burning sensation, and infusion site urticaria), Hypersensitvity reactions: (Hypotension, pyrexia, tremor, chills, urticaria, rash, and pruritus), Metabolism and nutrition disorders (Hypernatremia, hyponatremia, hyperchloremic metabolic acidosis) and Nervous System Disorders (Hyponatremic encephalopathy).
Do you have any questions or concerns?
Contact a Grifols representative for more information